- Using The Avogadro Number Aleks
- Using The Avogadro Number Aleks
- Using Avogadro's Number And Molar Masses
- Using The Avogadro's Number
- Using Avogadro's Number Calculator
Learning Objectives
- Use Avogadro's number to convert to moles and vice versa given the number of particles of an element.
- Know the definition of the mole.
- Determine the formula mass of an ionic or molecular compound.
- Determine the percent composition of each element in a compound from the chemical formula.
Avogadro's number is defined as the total number of entities in 12 g of carbon-12. N A = 6.022 × 10 23 It is a measuring criteria, just like a dozen, which is used to put a number to a certain item. A dozen means 12 items (items like bananas, atoms, molecules, etc) and 1 mole means 6.022 × 10 23 items (items like bananas, atoms, molecules, etc). 3) Use Avogadro's Number to determine number of molecules: (0.121076 mol) (6.022 x 10 23 molecules/mol) = 7.2912 x 10 22 molecules 4) Determine number of atoms. Number of atoms per mole (Ne - Yo Yow 7.159x103 7.224x10 Part 2: Estimation of Avogadro's number from aluminum foil. Measure the mass and area of your rectangular shaped piece of aluminum. Length (cm) width (cm) mass (8) ) 3.00 5.00 0.04600 Data Part 1 1. Was the estimation of Avogadro's number using steric acid accurate?
When objects are very small, it is often inconvenient or inefficient, or even impossible to deal with the objects one at a time. For these reasons, we often deal with very small objects in groups, and have even invented names for various numbers of objects. The most common of these is 'dozen' which refers to 12 objects. We frequently buy objects in groups of 12, like doughnuts or pencils. Even smaller objects such as straight pins or staples are usually sold in boxes of 144, or a dozen dozen. A group of 144 is called a 'gross'.

This problem of dealing with things that are too small to operate with as single items also occurs in chemistry. Atoms and molecules are too small to see, let alone to count or measure. Chemists needed to select a group of atoms or molecules that would be convenient to operate with.
Avogadro's Number:Counting Atoms
Owing to their tiny size, atoms and molecules cannot be counted by direct observation. But much as we do when 'counting' beans in a jar, we can estimate the number of particles in a sample of an element or compound if we have some idea of the volume occupied by each particle and the volume of the container. Once this has been done, we know the number of formula units (to use the most general term for any combination of atoms we wish to define) in any arbitrary weight of the substance. The number will of course depend both on the formula of the substance and on the weight of the sample. However, if we consider a weight of substance that is the same as its formula (molecular) weight expressed in grams, we have only one number to know: Avogadro's number.
Avogadro's number
Avogadro's number is known to ten significant digits:
[N_A = 6.022141527 times 10^{23}.]
However, you only need to know it to three significant figures:
[N_A approx 6.02 times 10^{23}. label{3.2.1}]
So (6.02 times 10^{23}) of what? Well, of anything you like: apples, stars in the sky, burritos. However, the only practical use for (N_A) is to have a more convenient way of expressing the huge numbers of the tiny particles such as atoms or molecules that we deal with in chemistry. Avogadro's number is a collective number, just like a dozen. Students can think of (6.02 times 10^{23}) as the 'chemist's dozen'.

Before getting into the use of Avogadro's number in problems, take a moment to convince yourself of the reasoning embodied in the following examples.
Things to understand about Avogadro's number
- It is a number, just as is 'dozen', and thus is dimensionless.
- It is a huge number, far greater in magnitude than we can visualize
- Its practical use is limited to counting tiny things like atoms, molecules, 'formula units', electrons, or photons.
- The value of NA can be known only to the precision that the number of atoms in a measurable weight of a substance can be estimated. Because large numbers of atoms cannot be counted directly, a variety of ingenious indirect measurements have been made involving such things as Brownian motion and X-ray scattering.
The Mole: 'A Dozen Eggs and a Mole of Sugar, Please'
The mole (symbol: mol) is the base unit of amount of substance ('number of substance') in the International System of Units or System International (SI), defined as exactly 6.02214076×1023 particles, e.g., atoms, molecules, ions or electrons. The current definition was adopted in November 2018, revising its old definition based on the number of atoms in 12 grams of carbon-12 (12C) (the isotope of carbon with relative atomic mass 12 Daltons by definition).
It is not obvious why eggs come in dozens rather than 10s or 14s, or why a ream of paper contains 500 sheets rather than 400 or 600. The definition of a mole—that is, the decision to base it on 12 g of carbon-12—is also arbitrary. The important point is that 1 mole of carbon—or of anything else, whether atoms, compact discs, or houses—always has the same number of objects: 6.02 × 1023.
The Mole
Video (PageIndex{1}) How big is a mole?
Converting Between Number of Atoms to Moles and Vice Versa
We can use Avogadro's number as a conversion factor, or ratio, in dimensional analysis problems. If we are given the number of atoms of an element X, we can convert it into moles of by using the relationship
[text{1 mol X} = 6.022 times 10^{23} text{ X atoms}.]
An example on the use of Avogadro's number as a conversion factor is given below for carbon.
Example (PageIndex{1}): Moles of Carbon
The element carbon exists in two primary forms: graphite and diamond. How many moles of carbon atoms is (4.72 times 10^{24}) atoms of carbon?
Solution
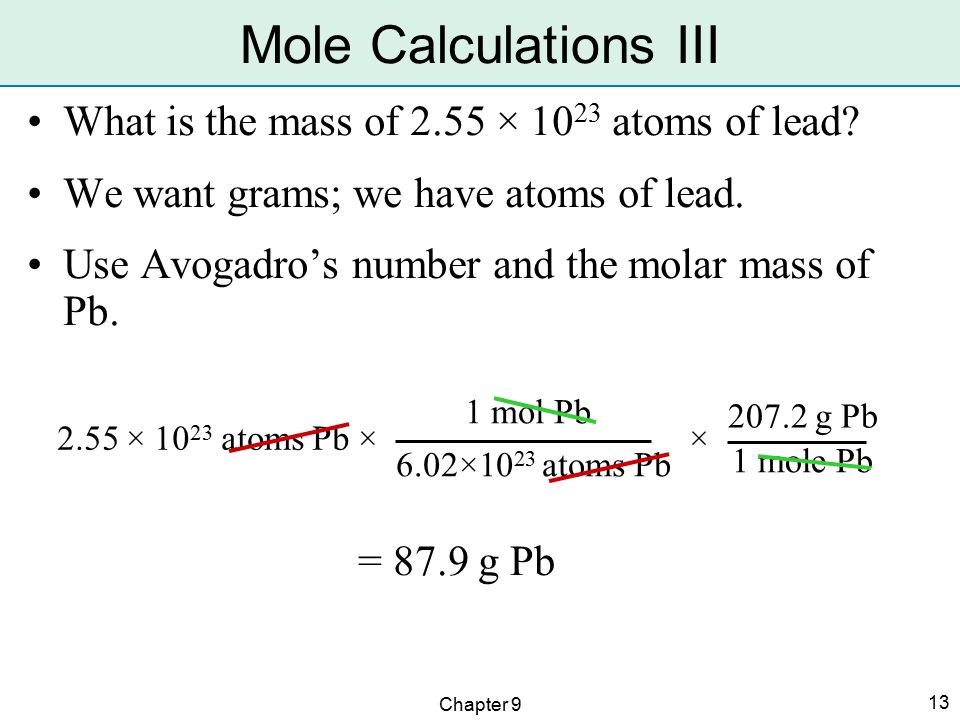
| Steps for Problem Solving | The element carbon exists in two primary forms: graphite and diamond. How many moles of carbon atoms is (4.72 times 10^{24}) atoms of carbon? |
|---|---|
| Identify the 'given'information and what the problem is asking you to 'find.' | Given: (4.72 times 10^{24}) C atoms Find: mol C |
| List other known quantities | (1, mol = 6.022 times 10^{23}) C atoms |
Prepare a concept map and use the proper conversion factor. | |
| Cancel units and calculate. | [4.72 times 10^{24} : cancel{text{C} : ce{atoms}} times frac{1 : text{mol} : ce{C}}{6.02 times 10^{23} : cancel{text{C} : ce{atoms}}} = 7.84 : text{mol} : ce{C} nonumber] |
| Think about your result. | The given number of carbon atoms was greater than Avogadro's number,so the number of moles of (ce{C}) atoms is greater than 1 mole. Since Avogadro's number is a measured quantity with three significant figures, the result of the calculation is rounded to three significant figures |
Formula Mass
One skill needed in future chapters is the ability to determine the mass of the formula of various chemical substances. This quantity is called the formula mass. The formula mass is obtained by adding the masses of each individual atom in the formula of the substance. Because a proper formula is electrically neutral (with no net electrons gained or lost), the ions can be considered atoms for the purpose of calculating the formula mass.
Let us start by calculating the formula mass of sodium chloride (NaCl). This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we find from the periodic table; here, we use the masses to two decimal places:
To two decimal places, the formula mass of NaCl is 58.44 amu.
For covalent substances, the formula represents the numbers and types of atoms composing a single molecule of the substance; therefore, the formula mass may be correctly referred to as a molecular mass. Consider chloroform (CHCl3), a covalent compound once used as a surgical anesthetic and now primarily used in the production of tetrafluoroethylene, the building block for the “anti-stick” polymer, Teflon. The molecular formula of chloroform indicates that a single molecule contains one carbon atom, one hydrogen atom, and three chlorine atoms. The average molecular mass of a chloroform molecule is therefore equal to the sum of the average atomic masses of these atoms.
For ionic compounds with polyatomic ions, the sum must include the number and mass of each atom in the formula for the polyatomic ion. as shown in the example below for aluminum sulfate, Al2(SO4)3.
Example (PageIndex{2}) Formula Mass for an Ionic Compound
Aluminum sulfate, Al2(SO4)3, is an ionic compound that is used in the manufacture of paper and in various water purification processes. What is the formula mass (amu) of this compound?
Solution
The formula for this compound indicates it contains Al3+ and SO42− ions combined in a 2:3 ratio. For purposes of computing a formula mass, it is helpful to rewrite the formula in the simpler format, Al2S3O12. Following the approach outlined above, the formula mass for this compound is calculated as follows:
The formula mass for Al2(SO4)3, is 342.14 amu.
Exercise (PageIndex{1})
Using The Avogadro Number Aleks
Use the atomic masses (rounded to two decimal places) to determine the formula mass for each ionic compound.
- TiO2
- AgBr
- Au(NO3)3
- Fe3(PO4)2
Answer
a. 79.87 amu
b. 187.77 amu
c. 383.0 amu
Percent Composition of a Compound from a Chemical Formula
The percent composition of a compound can also be determined from the formula of the compound. The subscripts in the formula are first used to calculate the mass of each element in one mole of the compound. That is divided by the molar mass of the compound and multiplied by (100%).
[% : text{by mass} = frac{text{mass of element in} : 1 : text{mol}}{text{molar mass of compound}} times 100%]
The percent composition of a given compound is always the same as long as the compound is pure.
Example (PageIndex{3})
Dichlorine heptoxide (left( ce{Cl_2O_7} right)) is a highly reactive compound used in some organic synthesis reactions. Calculate the percent composition of dichlorine heptoxide.
Solution
| Steps for Problem Solving | Calculate the percent composition of dichlorine heptoxide (left( ce{Cl_2O_7} right)). |
|---|---|
| Identify the 'given'information and what the problem is asking you to 'find.' | Given : Cl2O7 Find: % Composition (% Cl and %O) |
| List other known quantities | Mass of Cl in 1 mol Cl2O7 , 2 Cl : 2 x 35.45 g = 70.90 g Mass of O in 1 mol Cl2O7 , 7 O: 7 x 16.00 g = 112.00 g Molar mass of Cl2O7 = 182.90 g/mol |
| Cancel units and calculate. | [% ce{Cl} = frac{70.90 : text{g} : ce{Cl}}{182.90 : text{g}} times 100% = 38.76% : ce{Cl} nonumber] [% : ce{O} = frac{112.00 : text{g} : ce{O}}{182.90 : text{g}} times 100% = 61.24% : ce{O} nonumber] Calculate the percent by mass of each element by dividing the mass of that element in 1 mole of the compound by the molar mass of the compound and multiplying by (100%). |
| Think about your result. | The percentages add up to (100%). |
Percent composition can also be used to determine the mass of a certain element that is contained in any mass of a compound. In the previous sample problem, it was found that the percent composition of dichlorine heptoxide is (38.76% : ce{Cl}) and (61.24% : ce{O}). Suppose that you needed to know the masses of chlorine and oxygen present in a (12.50 : text{g}) sample of dichlorine heptoxide. You can set up a conversion factor based on the percent by mass of each element.
[12.50 : text{g} : ce{Cl_2O_7} times frac{38.76 : text{g} : ce{Cl}}{100 : text{g} : ce{Cl_2O_7}} = 4.845 : text{g} : ce{Cl}]
[12.50 : text{g} : ce{Cl_2O_7} times frac{61.24 : text{g} : ce{O}}{100 : text{g} : ce{Cl_2O_7}} = 7.655 : text{g} : ce{O}]
The sum of the two masses is (12.50 : text{g}), the mass of the sample size.
Exercise (PageIndex{2})
Barium fluoride is a transparent crystal that can be found in nature as the mineral frankdicksonite. Determine the percent composition of barium fluoride.
- Answer a:
- 78.32% Ba and 21.67% F
Summary
- The mole (symbol: mol) is the base unit of amount of substance ('number of substance') in the International System of Units or System International (SI), defined as exactly 6.02214076×1023 particles, e.g., atoms, molecules, ions or electrons.
- Avogadro's number is related to moles of any substance X as follows:
Using The Avogadro Number Aleks
[text{1 mol X} = 6.022 times 10^{23} text{ X atoms}.]
- Formula masses of ionic and molecular compounds can be determined from the masses of the atoms in their formulas.
- Processes are described for calculating the percent composition of a compound based on the chemical formula.
Anonymous
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
Stephen Lower, Professor Emeritus (Simon Fraser U.) Chem1 Virtual Textbook
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)
- Wikipedia
The value of Avogadro number (also known as Avogadro constant) is a positive natural integer as it indicates a finite and numerable quantity of elementary objects.
No constant, as well as no quantity of physics, can be defined with infinite certainty, therefore with arbitrary precision. On the subject of the precision of measurements it is always very interesting to refer to Heisenberg’s uncertainty principle and the epistemological, historical and philosophical influence it had in the scientific world when it was formalized.
What we will do in this article will be to define a purely theoretical value of Avogadro’s number, which is the value determined in the paper “An Equivalence for Both Coulombian and Gravitational Interactions”. This theoretical value will be compared to all the values of Avogadro’s number measured over time as well as the most modern measurements.
The theoretical determination of the Avogadro’s number
As stated, the paper “An Equivalence for Both Coulombian and Gravitational Interactions” defines the value of Avogadro’s number in relation with other natural constants. This expression of avogadro number is unprecedented in the history of physics. No one before has ever managed to express Avogadro’s number in relation to other natural constants. The expression of the Avogadro number (Avogadro constant) is :
Where:
- : Is the Avogadro number
- : Is the Language constantand its value is
- : Is the reduced Planck constant
- : Is the fine structure constant
- : Is the newtonian gravitation constant
- : Is the protonmass
- : Is the electronmass
Using Avogadro's Number And Molar Masses
As we can see from its expression, the Avogadro number indissolubly links the world of microscopic (particles) to the world of macroscopic (large gravitational masses).
This is certainly not only an extraordinary result but unique in the history of physics because nobody until 2019 had ever been able to understand how the quantum world of physics and the gravitational world were related and connected. In this description it strongly emerges that the Avogadro number indicates the amount of critical mass beyond which the world of microscopic becomes macroscopic. Avogadro’s number is the hinge between these two connected and connected worlds.
How much is the Avogadro number ?

The value officially accepted by the scientific community for the Avogadro constant is as follows:
Before continuing, it is important to define what the CODATA values are. The scientific community organizes its worldviews through a continuous and positive dialogue through all the experts of the individual branches of physics. Periodically all the experiments carried out in the world are analyzed and the results are sifted through. Following this global process of evaluating the entire world of laboratory experiments, the “most likely true” values are chosen for each of the characteristics of the observable universe. This is really beautiful work and high quality. Here you can find a direct link to CODATA 2018 value for the Avogadro number.
Using The Avogadro's Number
In 2018, the concept of kilograms, which has always been linked to the Avogadro number, was dealt with by the BIPM (Bureau International des Poids et Mesures) weights and measures commission in a special focus. Discussions on the subject can be found at this link: https://www.bipm.org/en/bipm/mass/avogadro/.
In the BIPM decision, a deterministic value is set for the Avogadro number so that all other constants in the international system are connected. This discussion and the related documents are really very interesting also to understand how the scientific community evolves.
This BIPM decision of 2018 has completely overturned the history of measurement.
For this reason for all calculations made with the proposed mathematical model we will use the CODATA 2014 values.
The experimental values of the Avogadro number
Here we propose to use the CODATA 2014 values or to take a look to all the historical measurement of the constant. The 2014 codata value is following:
Using Avogadro's Number Calculator
In his well written article “History and progress in the accurate determination of the Avogadro constant” the scientist Peter Becker makes a beautiful historical and quantitative analysis of evolution in the measure of Avogadro number. In his list he summarizes all the most important measures up to 2001.
Below we report a longer and more detailed research work summarized in a table produced by us for all measurements of the most important experiments.
We can observe how the more time passes the more the value of Avogadro’s number converges with the CODATA 2014 value we indicated.
In conclusion, we can say that we have explored the theme of Avogadro’s number and that we can observe that the value proposed for the constant is extremely accurate and in line with the experimental measurements, less an error of two parts per billion.
