- Smallest Particle Of An Element That Has The Characteristics Of That Element
- Smallest Part Of An Element
- Smallest Particle Of An Element With Properties Of That Element
The smallest particle of an element that retains its identity in a chemical reaction. A horizontal row of the periodic table. Daltons atomic theories. All matter is composed of extremely small particles called atoms 2. Atoms of a given element are. The smallest particle of a chemical element. Chemical element. Chemical element. Chemical element. Definition of atom. 1 a: the smallest particle of an element that can exist either alone or in combination an atom of hydrogen. B: the atom considered as a source of vast potential constructive or destructive energy a largely forgotten legacy of this country's conquest of the atom.
Contents
- Atomic number and Mass number
- Isotopes
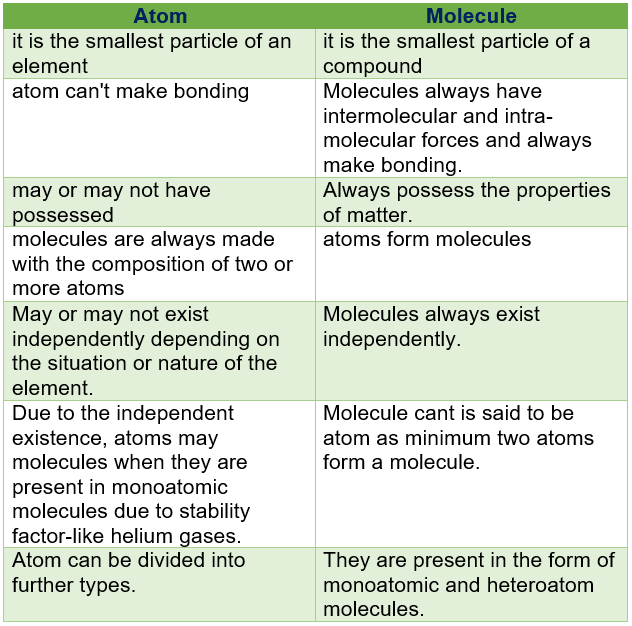
An atom is the smallest particle of an element still having the same chemical properties of the element. See the fact file below for more information on the atom or alternatively, you can download our 26-page Atom worksheet pack to utilise within the classroom or home environment. An atom is the smallest particle of any element, whereas, a molecule is formed when two or more atoms are held together with the help of a chemical bond. It is possible for atoms to either exist or not exist independently. However, molecules always exist independently or in a free state.
Atom
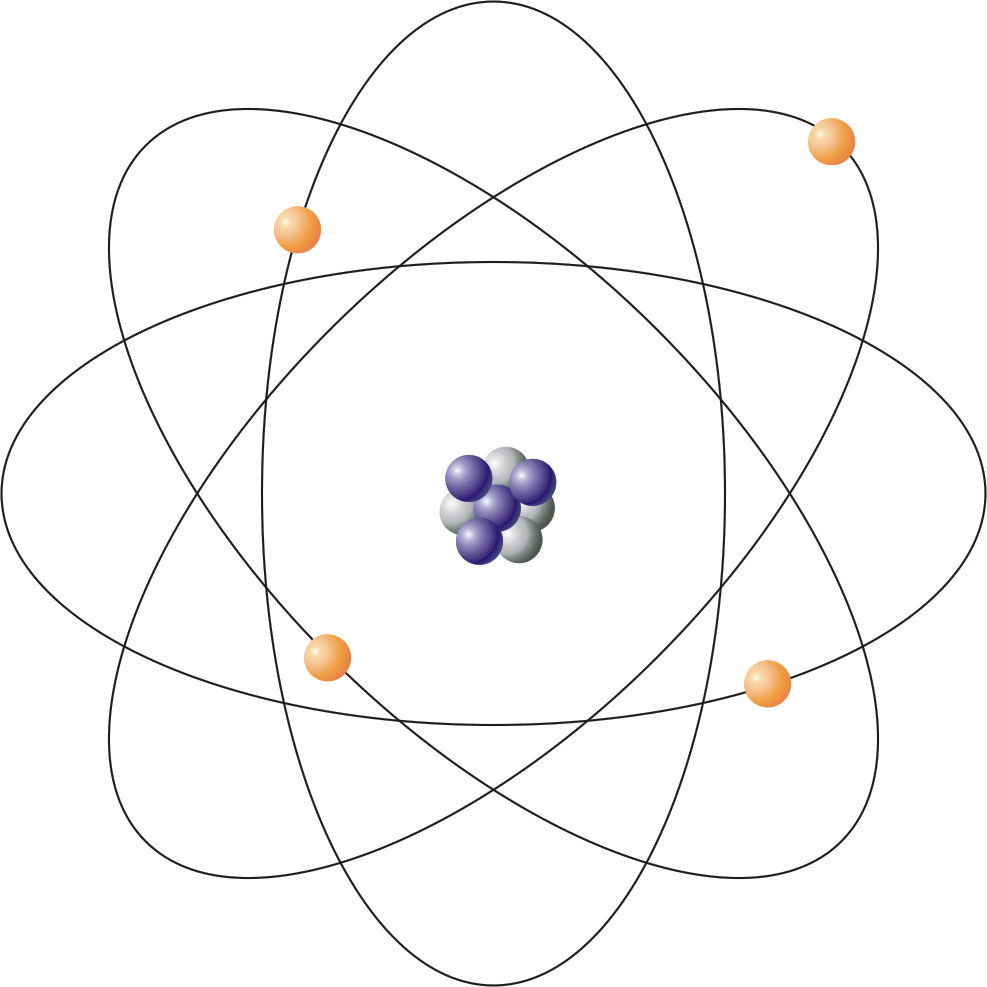
An atom is the smallest particle of an element which can take part in chemical reaction. Atom consists of three fundamental particles i.e. proton, neutron and electron. Atoms of same elements are similar in properties whereas atoms of different elements are different in properties. Example:- ‘H’ represent the atom of hydrogen.
Proton is positively charged and electron is negatively charged particle. In an atom, number of protons = number of electrons. Hence, the net charge present in an atom is zero i.e. a free atom is chargeless.
Atomic number and Mass number
Atomic number :
- Atomic number is the number of protons present in an atom.
- The modern periodic table is arranged in order of increasing atomic number.
Mass number and Atomic mass :
- Mass number is the sum of the number of protons and the number of neutrons present in an atom. It is a whole number.
Mass no. of an atom = No. of protons + No. of neutrons
- Atomic mass is the average mass of the all of the isotopes of that element. It is a decimal number.
- For example: Hydrogen has three isotopes – 1H1, 1H2 and 1H3 having mass number 1, 2 and 3 respectively. Naturally occurring hydrogen contains about 99.985% of protium, 0.014% of deuterium and 0.001 % of tritium. Therefore the atomic mass of hydrogen is 1.00784 amu.
- The atomic mass of an element element is measured in atomic mass unit (amu, also known as Daltons ‘ D’or unified atomic mass unit ‘u’).
- 1amu = 1.66 x 10-24 grams. 1gm = 6.022 x 1023 amu ( i.e. Avogadro’s number).
Here,
- Atomic number = Number of protons = Number of electrons = 13
- Mass number = No. of protons + No. of neutrons
- No. of neutrons = Mass number – No. of protons = 27-13 = 14.
Atomic mass of first 20 elements
| Atomic number | Element | Atomic mass |
| 1 | Hydrogen | 1.008 |
| 2 | Helium | 4.0026 |
| 3 | Lithium | 6.94 |
| 4 | Beryllium | 9.0122 |
| 5 | Boron | 10.81 |
| 6 | Carbon | 12.011 |
| 7 | Nitrogen | 14.007 |
| 8 | Oxygen | 15.999 |
| 9 | Fluorine | 18.998 |
| 10 | Neon | 20.180 |
| 11 | Sodium | 22.990 |
| 12 | Magnesium | 24.305 |
| 13 | Aluminium | 26.982 |
| 14 | Silicon | 28.085 |
| 15 | Phosphorus | 30.974 |
| 16 | Sulfur | 32.06 |
| 17 | Chlorine | 35.45 |
| 18 | Argon | 39.948 |
| 19 | Potassium | 39.098 |
| 20 | Calcium | 40.078 |
Isotopes
Atoms of the same element having same atomic number but different mass number (atomic mass/weight) are called isotopes. For example:
Isotopes of hydrogen :
There are three isotopes of hydrogen:
- Protium or ordinary hydrogen
- Deuterium or heavy hydrogen
- Tritium or radioactive hydrogen.
| Name | Protium | Deuterium | Tritium |
| Symbol | 1H or H | 2H or D | 3H or T |
| No. of protons(P) | 1 | 1 | 1 |
| No. of neutrons(n) | 0 | 1 | 2 |
| No. of electrons(e) | 1 | 1 | 1 |
| Atomic no.(Z) | 1 | 1 | 1 |
| Mass no.(A) | 1 | 2 | 3 |
Naturally occurring hydrogen contains about 99.985% of protium, 0.014% of deuterium and 0.001 % of tritium.
Isotopes have different physical properties since they differ in their mass number.
They have same chemical properties since their electronic configuration is same. However, they differ in the rate of chemical reaction. For example, D2 reacts with Cl2 about 13 times slower than H2 does. The different in rate of reaction due to difference in mass of the atoms of the same element is called isotope effect.
Some other examples of isotopic elements :
| Elements | Isotopes | Most abundant isotope |
| Carbon | 6C12, 6C13, 6C14 | 6C12 |
| Nitrogen | 7N14, 7N15 | 7N14 |
| Oxygen | 8O16, 8O17, 8O18 | 8O16 |
| Sulphur | 16S32, 16S33, 16S34, 16S36 | 16S32 |
| Chlorine | 17Cl35, 17S37 | 17Cl35 |
Isobars
Atoms of different elements having different atomic number but same mass number are called isobars. For example :
18Ar40, 19K40 and 20Ca40
Isotones
Atoms of different elements having different atomic number and mass number but same number of neutrons are called isotones. For example :
6C14, 7N15 and 8O16
Objective questions and their answers
1. Which of the following is known as heavy hydrogen?
a. Protium c. Tritium
b. Deuterium d. Para hydrogen
2. Which of the following is known as radioactive hydrogen?
Smallest Particle Of An Element That Has The Characteristics Of That Element
a. Protium c. Tritium
b. Deuterium d. Para hydrogen
3. Least abundant isotope of hydrogen is:

a. Protium c. Tritium
b. Deuterium d. Heavy hydrogen
4. Diamond and graphite are :
a. Isotopes c. Isotones
b. Isobars d. Allotropes
5. 6C14 and 8O16 are :
a. Isotopes c. Isotones
b. Isobars d. Allotropes
6. 6C14 and 7N14 are :
a. Isotopes c. Isotones

b. Isobars d. Allotropes
7. All particles residing inside the nucleus of an atom are termed as:
a. Protons c. Electrons
b. Neutrons d. Nucleons
8. What makes the atomic mass fractional ?
a.Prerence of isotopes
b. Number of unpaired electrons
c. Spherical shape
d. Quantum number.
9. Which of the following are not isotopes:
a. 1H1 and 1H3
b. 18K40 and 20Ca40
c. 6C14 and 7N14
d. Both b and c.
10. Charge present in the nucleus of an atom is :
a. Positive c. Chargeless
b. Negative d. Both +Ve and -Ve
11. Molecular weight of heavy water is :
a. 16 c. 20
b. 18 d. 22
Answers :
1. b 2. c 3. c
Smallest Part Of An Element
4. d [Note : different forms of same element having different properties are called allotropes]
5. c 6. b 7. d
8. a 9. d 10. a
11. c Note :Heavy water– Deuterium oxide (D2O) is called heavy water. It’s molecular weight is 20 and boiling paint is 101.50C and melting point is 3.80C.
References
Smallest Particle Of An Element With Properties Of That Element
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
