Enter the percentage abundance and mass of up to 5 different isotopes into the average atom mass calculator. The calculator will display the average atomic mass of the isotopes.
Density is the mass of a substance that would fill 1 cm 3 at room temperature. Relative atomic mass The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average. Atomic mass of Cobalt is 58.9332 u. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.

Average Atomic Mass Formula
The following formula is used to calculate the average atomic mass of a substance.
AM = f1M1 + f2M2 +… + fnMn
- Where AM is the average atomic mass
- fn is the fractional percent of the isotope
- M is the mass of the isotope
Atomic Mass Of Co3

Average Atomic Mass Definition
An average atomic mass is defined as the average mass of all isotopes within a given substance.
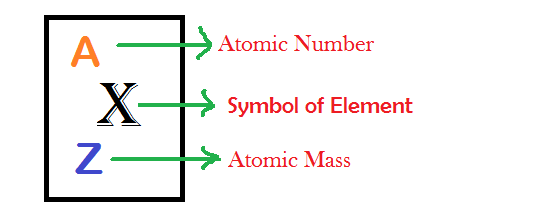
How to calculate average atomic mass?
How to calculate average atomic mass
- First, determine the fractional percent of each isotope in the substance
For example, chlorine has two major isotopes. 1 with 75.77 percent of atoms and 1 with 24.23 percent of atoms. These two percentages would be the fractional percents of those isotopes.
- Next, determine the masses of each isotope
Using the same example above, this would be 35 and 37 amu respectively.
- Finally, calculate the average atomic mass
Calculate the average atomic mass using the information from steps 1 and 2 and the formula above.
Atomic Mass Of Copper In Grams
FAQ
What is average atomic mass?The average atomic mass is the average mass of all of the isotopes that make up a substance.
Atomic Mass Of Co2
What is the fractional percent?Relative Atomic Mass Of Co
The fractional percent is the total percentage a particular isotope in a substance.
